5
Question
Answer the following question regarding the modern version of Mendeleev's periodic table.
How are the groups numbered?
Answer the following question regarding the modern version of Mendeleev's periodic table.
How are the groups numbered?
Open in App
Solution
In the modern version of Mendeleev's periodic table, groups are numbered in Roman numerals from I to VIII.
- In the modern version, another group called group-0 was also added so as not to disturb the entire periodic table.
- So the groups are numbered from 0 to VIII.
Groups: The vertical columns in Mendeleev's periodic table are known as groups.
- The number of groups present in the original Mendeleev's periodic table was 8 and later, in the modified form, another group called group-0 was added to the extreme left side of the periodic table.
- In Mendeleev's periodic table, elements were arranged in the order of the increasing atomic weights.
- The elements were arranged in the form of vertical columns called groups and horizontal rows called periods.
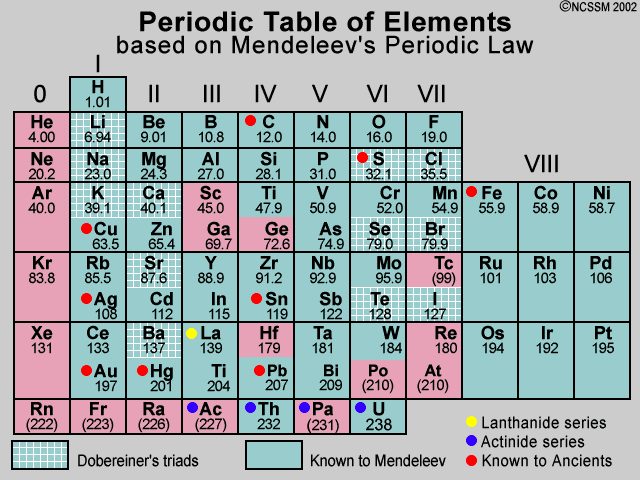
In the modern version of Mendeleev's periodic table, groups are numbered in Roman numerals from I to VIII.
- In the modern version, another group called group-0 was also added so as not to disturb the entire periodic table.
- So the groups are numbered from 0 to VIII.
Groups: The vertical columns in Mendeleev's periodic table are known as groups.
- The number of groups present in the original Mendeleev's periodic table was 8 and later, in the modified form, another group called group-0 was added to the extreme left side of the periodic table.
- In Mendeleev's periodic table, elements were arranged in the order of the increasing atomic weights.
- The elements were arranged in the form of vertical columns called groups and horizontal rows called periods.

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
