1
Question
Aqueous solution of borax reacts with two mol of acids. This is because of -
Aqueous solution of borax reacts with two mol of acids. This is because of -
Open in App
Solution
The correct option is D Formation of 2 mol each of 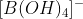 and
and 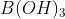 , of which only
, of which only 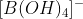 reacts with acid
reacts with acid
Na2B4O7+7H2O→2B(OH)3+2Na[B(OH)4]
B(OH)3orH3BO3 is an acid and does not react with acid.
Hence Na[B(OH)4] reacts with acid.
Formation of 2 mol each of and
, of which only
reacts with acid
Na2B4O7+7H2O→2B(OH)3+2Na[B(OH)4]
B(OH)3orH3BO3 is an acid and does not react with acid.
Hence Na[B(OH)4] reacts with acid.
