1
Question
Assertion :Benzene (C6H6) can be drawn as a series of resonance structures.
Reason: Its bonds are a hybrids of single and double bond character.
Assertion :Benzene (C6H6) can be drawn as a series of resonance structures.
Reason: Its bonds are a hybrids of single and double bond character.
Open in App
Solution
The correct option is A Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
Benzene can be drawn as a series of resonance structures, its bonds are hybrids of single and double bonds. The classical example in
resonance structures is the benzene ring. Benzene is a ring of 6 carbon atoms
with conjugated double bonds (i.e. alternating single and double bonds). When
drawing the ring, the double bonds can be located in 2 different positions as
shown in fig. 1. Both are equally right and wrong. The double bond between
position 1 and 2 is there some of the time, but on average there is really 1.5
bonds between all the carbons. The bond is said to be delocalized, which is why
the benzene ring is often written as a hexagon with a circle. The
difference from rearranging the electrons is called resonance structures, and
the change between structures is shown by a single arrow pointing both ways.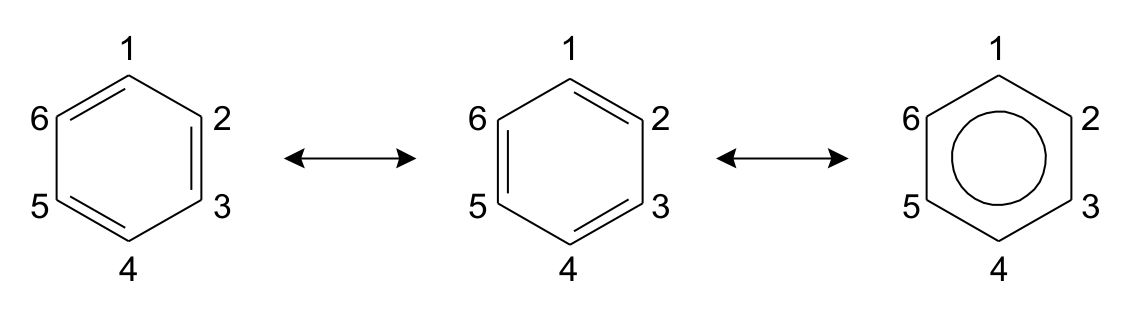
Benzene can be drawn as a series of resonance structures, its bonds are hybrids of single and double bonds. The classical example in resonance structures is the benzene ring. Benzene is a ring of 6 carbon atoms with conjugated double bonds (i.e. alternating single and double bonds). When drawing the ring, the double bonds can be located in 2 different positions as shown in fig. 1. Both are equally right and wrong. The double bond between position 1 and 2 is there some of the time, but on average there is really 1.5 bonds between all the carbons. The bond is said to be delocalized, which is why the benzene ring is often written as a hexagon with a circle. The difference from rearranging the electrons is called resonance structures, and the change between structures is shown by a single arrow pointing both ways.

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
