1
Question
Briefly describe the experiment which led to the discovery of nucleus.
Briefly describe the experiment which led to the discovery of nucleus.
Open in App
Solution
- Discovery of the nucleus within an atom is attributed to Ernest Rutherford.
- Rutherford performed rays scattering experiment.
Rays scattering experiment-
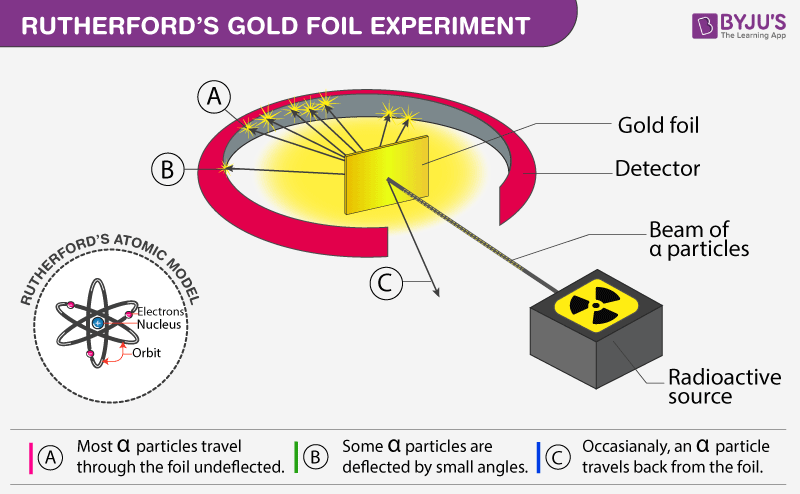
- Rutherford bombarded a thin gold foil with fast-moving alpha particles from a radioactive source, Polonium.
- Each alpha particle has two protons and two neutrons,.
- A movable circular zinc sulfide screen was placed after the gold foil to detect the deflections suffered by alpha particles.
Observations-
- Most of the alpha particles could pass undeflected through the gold foil.
- Some particles were deflected slightly from their path.
- A very few particles bounced back from their original path.
Explanation-
- According to Rutherford following conclusions were made.
- There is a large empty space in the gold atom due to which most of the alpha particles could pass.
- The central part of the atom is heavy and positively charged which caused the deflection of a few particles.
- This positively charged central part was the nucleus.
- Discovery of the nucleus within an atom is attributed to Ernest Rutherford.
- Rutherford performed rays scattering experiment.
Rays scattering experiment-
- Rutherford bombarded a thin gold foil with fast-moving alpha particles from a radioactive source, Polonium.
- Each alpha particle has two protons and two neutrons,.
- A movable circular zinc sulfide screen was placed after the gold foil to detect the deflections suffered by alpha particles.

Observations-
- Most of the alpha particles could pass undeflected through the gold foil.
- Some particles were deflected slightly from their path.
- A very few particles bounced back from their original path.
Explanation-
- According to Rutherford following conclusions were made.
- There is a large empty space in the gold atom due to which most of the alpha particles could pass.
- The central part of the atom is heavy and positively charged which caused the deflection of a few particles.
- This positively charged central part was the nucleus.
Suggest Corrections
6
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
