1
Question
Draw the Sawhorse and Newman projections for the staggered and eclipsed conformations of ethane.
Discuss their relative stability. Can these conformations bne separated ? If not, then why?
Discuss their relative stability. Can these conformations bne separated ? If not, then why?
Open in App
Solution
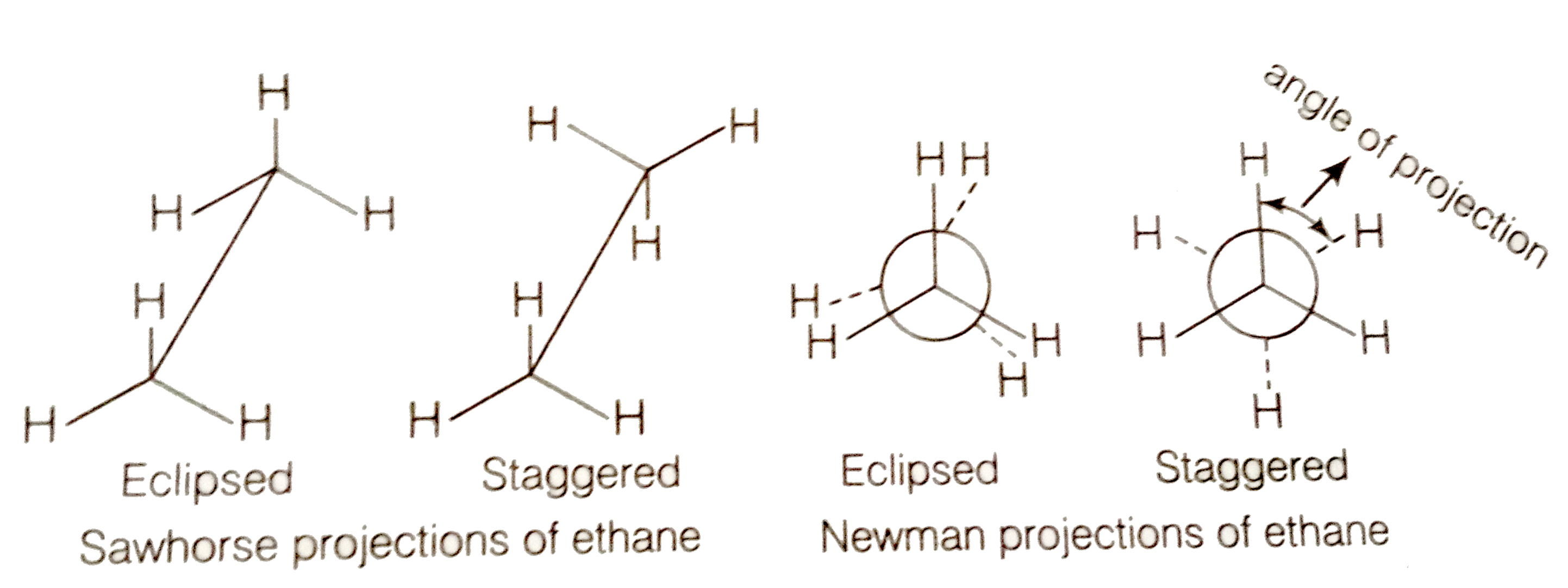
Staggered form of ethane is more stable than the eclipsed conformation by about 12.55 KJ/mol. This is because any two hydrogen atoms on adjacent carbon atoms of staggered conformation are maximum apart while in eclipsed conformation, they cover or eclipse each other in space. Thus in staggered form, there is minimum repulsive forces, minimum energy and maximum stability of the molecule.

Staggered form of ethane is more stable than the eclipsed conformation by about 12.55 KJ/mol. This is because any two hydrogen atoms on adjacent carbon atoms of staggered conformation are maximum apart while in eclipsed conformation, they cover or eclipse each other in space. Thus in staggered form, there is minimum repulsive forces, minimum energy and maximum stability of the molecule.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
