1
Question
What is an ideal gas? Explain.
What is an ideal gas? Explain.
Open in App
Solution
Explanation
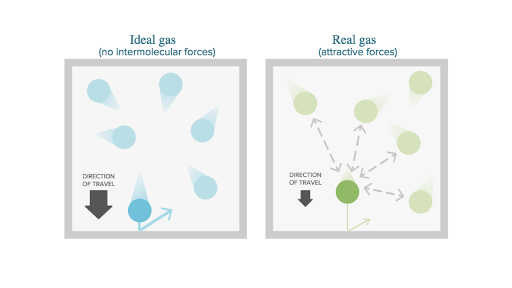
- An ideal gas is a theoretical gas with zero inter-molecular force between many randomly moving point particles.
- Point particles are point mass with negligible volume. They do not attract or repel the adjacent molecules.
- In ideal gas is collisions between the inter molecules or inter atoms are perfectly elastic as well as there’s no intermolecular force of attraction
- Ideal gases follow a simplified equation of state called as ideal gas law. It is represented by four variables P, V, T as well as n constant.
- Ideal gas law relates volume of the gas (V), its pressure (P),to the number of moles of gas (n) and temperature (T) and ideal gas constant, R as
Explanation
- An ideal gas is a theoretical gas with zero inter-molecular force between many randomly moving point particles.
- Point particles are point mass with negligible volume. They do not attract or repel the adjacent molecules.
- In ideal gas is collisions between the inter molecules or inter atoms are perfectly elastic as well as there’s no intermolecular force of attraction
- Ideal gases follow a simplified equation of state called as ideal gas law. It is represented by four variables P, V, T as well as n constant.
- Ideal gas law relates volume of the gas (V), its pressure (P),to the number of moles of gas (n) and temperature (T) and ideal gas constant, R as

Suggest Corrections
1
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
