1
Question
Explain the structure of carbon dioxide.
Explain the structure of carbon dioxide.
Open in App
Solution
Structure of Carbon Dioxide
- The carbon dioxide molecule has two double bonds between Carbon and Oxygen atoms.
- A double bond consists of a sigma and a pi bond which means in total contains sigma and pi bonds.
- The bond angle between Carbon and two Oxygen is 180° as it is linear in structure.
- The hybridization of in is and is hybridized.
- With the electronegativity difference in and, the bonds formed in are polar.
- But because of two Oxygen molecules on each side of Carbon, the dipole moment gets canceled, and the compound has no final dipole moment.

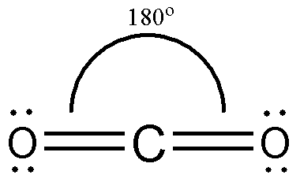
Structure of Carbon Dioxide
- The carbon dioxide molecule has two double bonds between Carbon and Oxygen atoms.
- A double bond consists of a sigma and a pi bond which means in total contains sigma and pi bonds.
- The bond angle between Carbon and two Oxygen is 180° as it is linear in structure.
- The hybridization of in is and is hybridized.
- With the electronegativity difference in and, the bonds formed in are polar.
- But because of two Oxygen molecules on each side of Carbon, the dipole moment gets canceled, and the compound has no final dipole moment.


Suggest Corrections
25
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
