6
Question
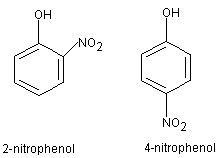
Give the type of isomerism exhibited by the following pairs.o - Nitrophenol and m-nitrophenol
o - Nitrophenol and m-nitrophenol
Open in App
Solution
The correct option is A Positional
o - Nitrophenol and m-nitrophenol exbhit Positional isomerism.The positional isomerism arises due to different positions of side chains, substituents, functional groups, double bonds, triple bonds etc., on the parent chain.
Hence option A is correct.
o - Nitrophenol and m-nitrophenol exbhit Positional isomerism.
The positional isomerism arises due to different positions of side chains, substituents, functional groups, double bonds, triple bonds etc., on the parent chain.

Hence option A is correct.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
