1
Question
In a source of electricity such as simple voltaic cell, the electrode at higher potential is made of
In a source of electricity such as simple voltaic cell, the electrode at higher potential is made of
Open in App
Solution
The correct option is A Copper
An electrolytic cell is an electrochemical cell that drives a non-spontaneous redox reaction through the application of electrical energy. They are often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means to break up.Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chemicals. Electroplating (e.g. of copper, silver, nickel or chromium) is done using an electrolytic cell. Electrolysis is a technique that uses a direct electric current (DC).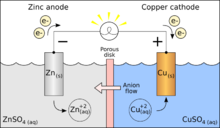 In a source of electricity such as simple voltaic cell, the electrode at higher potential is made of Copper(Cu)
In a source of electricity such as simple voltaic cell, the electrode at higher potential is made of Copper(Cu)
An electrolytic cell is an electrochemical cell that drives a non-spontaneous redox reaction through the application of electrical energy. They are often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means to break up.Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chemicals. Electroplating (e.g. of copper, silver, nickel or chromium) is done using an electrolytic cell. Electrolysis is a technique that uses a direct electric current (DC).

In a source of electricity such as simple voltaic cell, the electrode at higher potential is made of Copper(Cu)
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
