1
Question
In which of the following equilibria, the value of KP is less than KC
In which of the following equilibria, the value of KP is less than KC
Open in App
Solution
The correct option is B N2 + 3H2 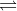 2NH3
2NH3
For the reaction: N2 + 3H2 ⇌ 2NH3
KP = KC(RT)△n; When △n = 2 − (2 + 1) = −1, i.e. negative. Hence KP < KC
N2 + 3H2 2NH3
For the reaction: N2 + 3H2 ⇌ 2NH3
KP = KC(RT)△n; When △n = 2 − (2 + 1) = −1, i.e. negative. Hence KP < KC
Suggest Corrections
5
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
