1
Question
In which of the following reaction, the value of Kp will be equal to Kc
In which of the following reaction, the value of Kp will be equal to Kc
Open in App
Solution
The correct option is A H2 + I2 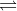 2HI
2HI
if np = nr then KP = KC
where np = no. of moles of product
nr = no. of moles of reactant.
H2 + I2 2HI
if np = nr then KP = KC
where np = no. of moles of product
nr = no. of moles of reactant.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
