1
Question
Indicate the correct statement for a 1-L sample of N2(g) and CO2(g) at 298 K and 1 atm pressure
Indicate the correct statement for a 1-L sample of N2(g) and CO2(g) at 298 K and 1 atm pressure
Open in App
Solution
The correct options are
A The average translational KE per molecule is the same in 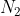 and
and 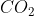
C The density of 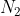 is less than that of
is less than that of 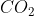
D The total translational KE of both 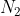 and
and 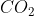 is the same
is the same
Kinetic energies per molecule will be same because it is proportional to absolute temperature only.
dN2dCO2=MN2MCO2=2844i.e.dN2<dCO2
Total translational kinetic energy will also be same because at same temperature & pressure number of molecules present in same volume would be same (according to Avogadro's Law)
A
The average translational KE per molecule is the same in and
C
The density of is less than that of
D
The total translational KE of both and
is the same
Kinetic energies per molecule will be same because it is proportional to absolute temperature only.
dN2dCO2=MN2MCO2=2844i.e.dN2<dCO2
Total translational kinetic energy will also be same because at same temperature & pressure number of molecules present in same volume would be same (according to Avogadro's Law)
