1
Question
An ionic bond is present in which of the following species:
An ionic bond is present in which of the following species:
Open in App
Solution
The correct option is A Sodium Bromide
Explanation for Correct Option:
Option (A): Sodium Bromide
- An ionic bond is defined as the attractive electrostatic force that is present between two oppositely charged ions.
- The formation of an ionic bond occurs when excess electrons from an anion are transferred to a cation in order to attain their nearest inert gas configuration.
- In Sodium bromide the cation is and the anion is .
- Hence, they have oppositely charged ions that form an ionic bond through electrostatic force of attraction.
Explanation for Incorrect Option:
Option (C) Hydrogen Chloride
- In, Hydrogen chloride, the Hydrogen atom shares its 1 electron with Chlorine, and thus forms a covalent bond.
- Hence, they do not form ionic bonds.

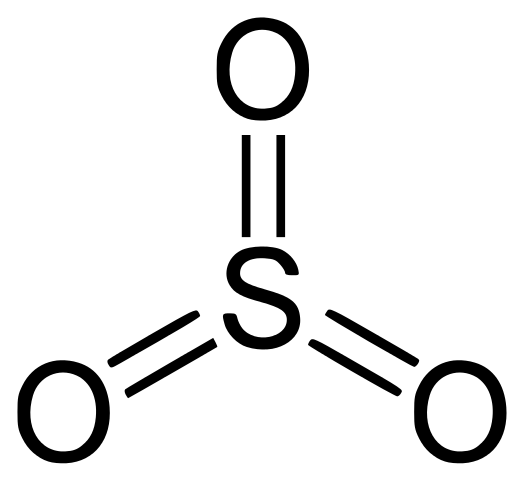
Option (B): Sulfur Trioxide
- In Sulfur trioxide both Sulphur and Oxygen form a covalent bond.
- Hence, they do not form ionic bonds.
Option (D): Carbon Monoxide
- In Carbon monoxide both Carbon and Oxygen form a covalent bond,
- Hence, they do not form ionic bonds.

Therefore, an ionic bond is present in which of the following species option (A): Sodium Bromide
Sodium Bromide
Explanation for Correct Option:
Option (A): Sodium Bromide
- An ionic bond is defined as the attractive electrostatic force that is present between two oppositely charged ions.
- The formation of an ionic bond occurs when excess electrons from an anion are transferred to a cation in order to attain their nearest inert gas configuration.
- In Sodium bromide the cation is and the anion is .
- Hence, they have oppositely charged ions that form an ionic bond through electrostatic force of attraction.
Explanation for Incorrect Option:
Option (C) Hydrogen Chloride
- In, Hydrogen chloride, the Hydrogen atom shares its 1 electron with Chlorine, and thus forms a covalent bond.
- Hence, they do not form ionic bonds.

Option (B): Sulfur Trioxide
- In Sulfur trioxide both Sulphur and Oxygen form a covalent bond.
- Hence, they do not form ionic bonds.

Option (D): Carbon Monoxide
- In Carbon monoxide both Carbon and Oxygen form a covalent bond,
- Hence, they do not form ionic bonds.

Therefore, an ionic bond is present in which of the following species option (A): Sodium Bromide
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
