London dispersion force is the only intermolecular force that works on what?
London dispersion force is the only intermolecular force that works on what?
The correct option is C Noble gases and nonpolar molecules
(A) : Noble gas and non-polar molecules.
The London dispersion force is the weakest intermolecular force.
The London dispersion force is a temporary attractive force that results
when the electrons in two adjacent atoms occupy positions that make the
atoms form temporary dipoles. This force is sometimes called an induced
dipole-induced dipole attraction. London forces are the attractive
forces that cause nonpolar substances to condense to liquids and to freeze
into solids when the temperature is lowered sufficiently.
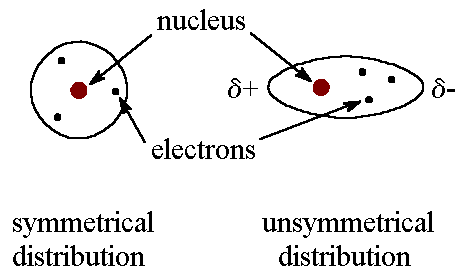
Because of the constant motion of the electrons, an atom or molecule
can develop a temporary (instantaneous) dipole when its electrons are distributed
unsymmetrically about the nucleus.
(A) : Noble gas and non-polar molecules.

The London dispersion force is the weakest intermolecular force. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction. London forces are the attractive forces that cause nonpolar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently.
Because of the constant motion of the electrons, an atom or molecule can develop a temporary (instantaneous) dipole when its electrons are distributed unsymmetrically about the nucleus.
