92
Question
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
Open in App
Solution
The correct option is A Addition Reaction
Explanation for correct answer
Option (A) Addition Reaction
- Fats are formed when oils are treated with hydrogen in the presence of a palladium or nickel catalyst.
- Hydrogen is added to oil by reducing agents palladium or nickel, this is called as Addition reactions.
- Chemical reaction involved:
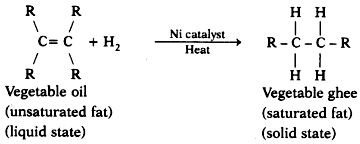
- Hence option (A) is correct.
Explanation for incorrect answer
Option (B) Substitution Reaction
- A substitution reaction is a chemical reaction in which one functional group in a chemical compound is replaced by another functional group.
- Hence, in this Hydrogen is adding not replacing, hence option (B) is incorrect.
Option (C) Displacement reaction
- It is a chemical reaction in which one element is replaced by another in a compound.
- Hence, in this Hydrogen is adding, so option (C) is incorrect.
Option (D) Oxidation reaction
- In a chemical reaction, when the loss of electrons or increase in oxidation state of a chemical atoms in it, called as Oxidation reaction.
- Hence in this Hydrogen is added so reduction takes place.
- Hence option (D) is incorrect.
Therefore, addition of Hydrogen is taking place so it an example of addition reaction. Hence option (A) is correct.
Addition Reaction
Explanation for correct answer
Option (A) Addition Reaction
- Fats are formed when oils are treated with hydrogen in the presence of a palladium or nickel catalyst.
- Hydrogen is added to oil by reducing agents palladium or nickel, this is called as Addition reactions.
- Chemical reaction involved:
- Hence option (A) is correct.
Explanation for incorrect answer
Option (B) Substitution Reaction
- A substitution reaction is a chemical reaction in which one functional group in a chemical compound is replaced by another functional group.
- Hence, in this Hydrogen is adding not replacing, hence option (B) is incorrect.
Option (C) Displacement reaction
- It is a chemical reaction in which one element is replaced by another in a compound.
- Hence, in this Hydrogen is adding, so option (C) is incorrect.
Option (D) Oxidation reaction
- In a chemical reaction, when the loss of electrons or increase in oxidation state of a chemical atoms in it, called as Oxidation reaction.
- Hence in this Hydrogen is added so reduction takes place.
- Hence option (D) is incorrect.
Therefore, addition of Hydrogen is taking place so it an example of addition reaction. Hence option (A) is correct.
Suggest Corrections
1
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
