1
Question
Resonance is not exhibited by................…?
Resonance is not exhibited by................…?
Open in App
Solution
The correct option is D Cyclohexane
Explanation for the correct option:
Option(D):Cyclohexane
- Resonance is generally known to be observed in the compounds having alternate single and multiple bonds i.e conjugated compounds.
- The actual structure of any given compound is known to be the resonance hybrid of various possible alternate structures which are known as resonating structures or canonical structures.
- Hence, to show resonance effect the compounds must contain the delocalization of pi electrons or unshared electrons without shifting any atom.
- From the structures of Cyclohexane, we can conclude that it does not have any delocalized conjugated pi-electron system, hence it does not show any resonance.

The explanation for the incorrect option:
Option(A):Phenol
- Phenol is generally known to exhibit Resonance, as it has the delocalized conjugated pi-electron system, which can be shown as:
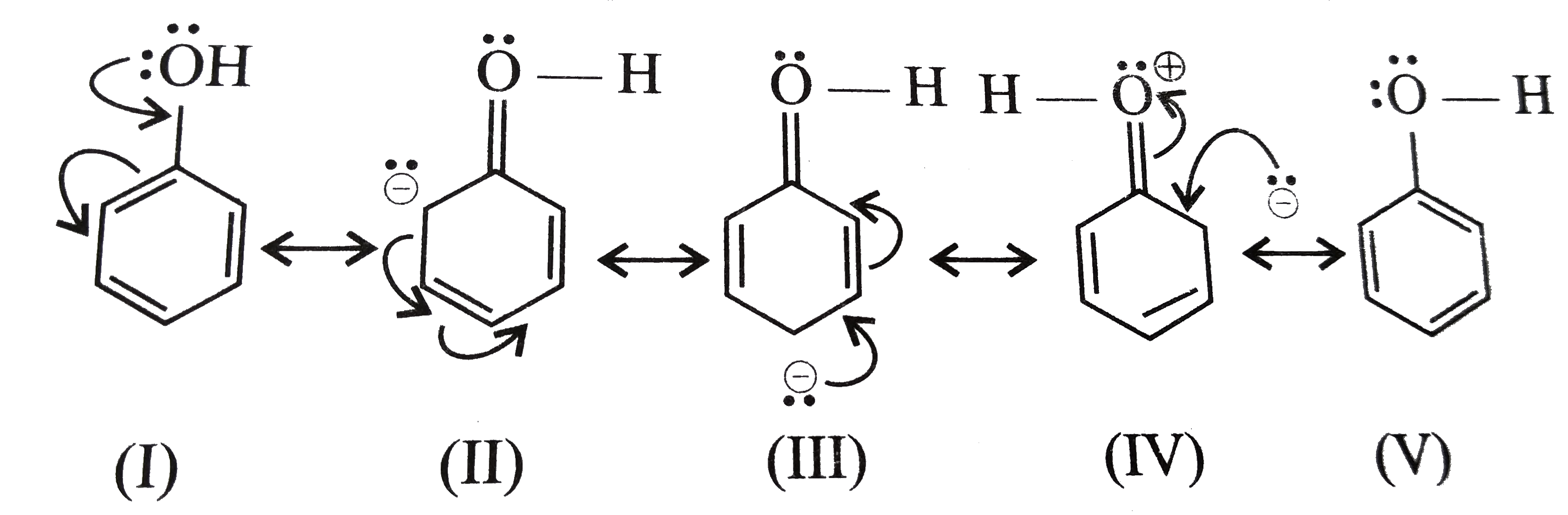
![]()
- Hence, it shows resonance.
Option(B):Aniline
- Resonance can be exhibited by Aniline, as it is a conjugated molecule, which can be shown as:

- Hence, it shows resonance.
Option(C):Nitrobenzene
- Nitrobenzene is generally known to exhibit Resonance, as it has the delocalized conjugated pi-electron system, which can be shown as:

- Hence, it shows resonance.
Hence, Resonance is not exhibited by Cyclohexane and the correct option is (D).
Cyclohexane
Explanation for the correct option:
Option(D):Cyclohexane
- Resonance is generally known to be observed in the compounds having alternate single and multiple bonds i.e conjugated compounds.
- The actual structure of any given compound is known to be the resonance hybrid of various possible alternate structures which are known as resonating structures or canonical structures.
- Hence, to show resonance effect the compounds must contain the delocalization of pi electrons or unshared electrons without shifting any atom.
- From the structures of Cyclohexane, we can conclude that it does not have any delocalized conjugated pi-electron system, hence it does not show any resonance.

The explanation for the incorrect option:
Option(A):Phenol
- Phenol is generally known to exhibit Resonance, as it has the delocalized conjugated pi-electron system, which can be shown as:

- Hence, it shows resonance.
Option(B):Aniline
- Resonance can be exhibited by Aniline, as it is a conjugated molecule, which can be shown as:
- Hence, it shows resonance.
Option(C):Nitrobenzene
- Nitrobenzene is generally known to exhibit Resonance, as it has the delocalized conjugated pi-electron system, which can be shown as:
- Hence, it shows resonance.
Hence, Resonance is not exhibited by Cyclohexane and the correct option is (D).
Suggest Corrections
2
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
