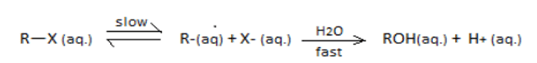1
Question
SN1 reaction undergoes through a carbocation intermediate as follows:
 [R=t−Bu,iso−Pr,Et,Me](X=Cl,Br,I)
[R=t−Bu,iso−Pr,Et,Me](X=Cl,Br,I)
The correct statements are
(I) The decreasing order of rate of SN1 reaction is t−BuX>iso−PrX>EtX>MeX
(II) The decreasing order of ionization energy is
MeX>EtX>iso−PrX>t−BuX
(III) The decreasing order of energy of activation is t−BuX>iso−PrX>EtX>MeX
 [R=t−Bu,iso−Pr,Et,Me](X=Cl,Br,I)
[R=t−Bu,iso−Pr,Et,Me](X=Cl,Br,I)The correct statements are
(I) The decreasing order of rate of SN1 reaction is t−BuX>iso−PrX>EtX>MeX
(II) The decreasing order of ionization energy is
MeX>EtX>iso−PrX>t−BuX
(III) The decreasing order of energy of activation is t−BuX>iso−PrX>EtX>MeX
Open in App
Solution
The correct option is A I and II are correct
In SN1 reaction the ionization of -X group to form a carbo cation is the first and rate determining reaction.
Among the given halides, higher the stability of the cabocation formed i) lower the ionization energy of the -C-X bond ii) lower the energy of activation for substition by -OX and iii) faster the substitution reaction.
Stability of the carboctions follow the order t−BuX>iso−PrX>EtX>MeX
So the order for:
I) Rate of SN1 reaction is a
t−BuX>iso−PrX>EtX>MeX
II) Order of ionisation energy
t−BuX<iso−PrX<EtX<MeX
III) Order of activation energy
t−BuX<iso−PrX<EtX<MeXand
Option a) is the correct option
In SN1 reaction the ionization of -X group to form a carbo cation is the first and rate determining reaction.
Among the given halides, higher the stability of the cabocation formed i) lower the ionization energy of the -C-X bond ii) lower the energy of activation for substition by -OX and iii) faster the substitution reaction.
Stability of the carboctions follow the order t−BuX>iso−PrX>EtX>MeX
So the order for:
I) Rate of SN1 reaction is a
t−BuX>iso−PrX>EtX>MeX
II) Order of ionisation energy
t−BuX<iso−PrX<EtX<MeX
III) Order of activation energy
t−BuX<iso−PrX<EtX<MeXand
Option a) is the correct option
Suggest Corrections
1
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program