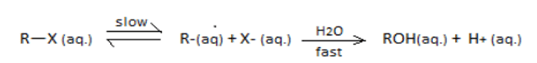1
Question
SN1 reaction undergoes through a carbocation intermediate as follows:
R−X(aq)slow⇌R+(aq)+X−(aq)H2O−−−→FastROH(aq)+H+(aq)
[R=t−Bu, iso−Pr, Et, Me]
Choose the correct statement(s):
R−X(aq)slow⇌R+(aq)+X−(aq)H2O−−−→FastROH(aq)+H+(aq)
[R=t−Bu, iso−Pr, Et, Me]
Choose the correct statement(s):
Open in App
Solution
The correct option is B The decreasing order of ionization energy is: MeX>EtX>iso−PrX>t−BuX
Rate of reaction of SN1∝Stability of carbocation
Stability of carbocation∝Number of α−H
∴The decreasing order of rate of SN1 reaction is:
t−BuX>iso−PrX>EtX>MeX
Ionization energy∝1Stability of carbocation
∴The decreasing order of rate of ionization energy is:
MeX>EtX>iso−PrX>t−BuX
Activation energy∝1Stability of carbocation
∴ The decreasing order of activation energy is:
MeX>EtX>iso−PrX>t−BuX
Hence, statements (a) and (b) are correct.
Rate of reaction of SN1∝Stability of carbocation
Stability of carbocation∝Number of α−H
∴The decreasing order of rate of SN1 reaction is:
t−BuX>iso−PrX>EtX>MeX
Ionization energy∝1Stability of carbocation
∴The decreasing order of rate of ionization energy is:
MeX>EtX>iso−PrX>t−BuX
Activation energy∝1Stability of carbocation
∴ The decreasing order of activation energy is:
MeX>EtX>iso−PrX>t−BuX
Hence, statements (a) and (b) are correct.
Suggest Corrections
2
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program