1
Question
SN I reaction undergoes through a carbocation intermediate as follows :
R−X(aq.)Slow⇌R∗(aq.)+X−(aq.)H2O−−→fastROH(aq.)+H+(aq.)
[R = t-Bu, iso-Pr, Et, Me] (X = Cl, Br, I)
The correct statements are
(I) The decreasing order of rate of SN 1 reaction is t-BuX > iso-PrX > EtX > MeX
(II) The decreasing order of ionization energy is MeX > EtX > iso-PrX> t-BuX
(III) The decreasing order of energy of activation is t-BuX > iso-PrX > EtX > MeX
R−X(aq.)Slow⇌R∗(aq.)+X−(aq.)H2O−−→fastROH(aq.)+H+(aq.)
[R = t-Bu, iso-Pr, Et, Me] (X = Cl, Br, I)
The correct statements are
(I) The decreasing order of rate of SN 1 reaction is t-BuX > iso-PrX > EtX > MeX
(II) The decreasing order of ionization energy is MeX > EtX > iso-PrX> t-BuX
(III) The decreasing order of energy of activation is t-BuX > iso-PrX > EtX > MeX
Open in App
Solution
The correct option is D I, II and III are correct
Rate of SN1 reaction is a
t – Bu X > iso – PrX > EtX > MeX
So order of activation energy
t – Bu X > iso – PrX > EtX > MeX
and order of ionization energy
t – BuX < iso – PrX < EtX < MeX
Rate of SN1 reaction is a
t – Bu X > iso – PrX > EtX > MeX
So order of activation energy
t – Bu X > iso – PrX > EtX > MeX
and order of ionization energy
t – BuX < iso – PrX < EtX < MeX
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program


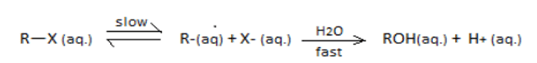
 Choose the correct decreasing order of stablity:
Choose the correct decreasing order of stablity: