1
Question
Starting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is W1 if the process is purely isothermal, W2 if purely isobaric and W3 if purely adiabatic. Then
Starting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is W1 if the process is purely isothermal, W2 if purely isobaric and W3 if purely adiabatic. Then
Open in App
Solution
The correct option is A W2>W1>W3
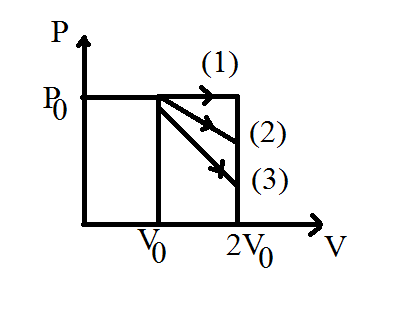 Explanation :Curve 1 - isobaric processCurve 2 - isothermal processCurve 3 - idiabatic processSince work done Area under PV graph=W2>W1>W3
Explanation :Curve 1 - isobaric processCurve 2 - isothermal processCurve 3 - idiabatic processSince work done Area under PV graph=W2>W1>W3
Explanation :
Curve 1 - isobaric process
Curve 2 - isothermal process
Curve 3 - idiabatic process
Since work done Area under PV graph
=W2>W1>W3
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program


