1
Question
Starting with the same initial conditions, one mole of an ideal monoatomic gas expands reversibly from volume V1 to V2 in two different ways. The work done by gas is w1, if the process is purely isothermal, w2 if purely isobaric and w3 if purely adiabatic, then:
Starting with the same initial conditions, one mole of an ideal monoatomic gas expands reversibly from volume V1 to V2 in two different ways. The work done by gas is w1, if the process is purely isothermal, w2 if purely isobaric and w3 if purely adiabatic, then:
Open in App
Solution
The correct option is A w2>w1>w3
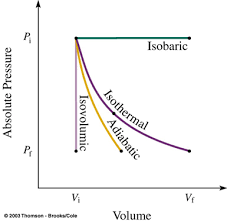 W =−∫PdVIt is the area under p-V diagram
W =−∫PdVIt is the area under p-V diagram
From the above image, we can say that the area under the P-V curve is in the increasing order: adiabatic, isothermal and isobaric
Hence, w2>w1>w3
W =−∫PdV
It is the area under p-V diagram
From the above image, we can say that the area under the P-V curve is in the increasing order: adiabatic, isothermal and isobaric
Hence, w2>w1>w3
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program

