1
Question
Sulphur dioxide gas turns acidified potassium dichromate paper_____.
Sulphur dioxide gas turns acidified potassium dichromate paper_____.
Open in App
Solution
The correct option is B green
Potassium dichromate (H2Cr2O7) is orange in colour. Oxidization state of Cr is +6 in potassium dichromate.
when it reacts with 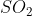 and
and 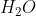 the following reaction takes place
the following reaction takes place

Formation of  takes place in which oxidation state of chromium is +3 and orange colour changes to green.
takes place in which oxidation state of chromium is +3 and orange colour changes to green.
Potassium dichromate (H2Cr2O7) is orange in colour. Oxidization state of Cr is +6 in potassium dichromate.
when it reacts with 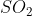 and
and 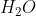 the following reaction takes place
the following reaction takes place
Formation of  takes place in which oxidation state of chromium is +3 and orange colour changes to green.
takes place in which oxidation state of chromium is +3 and orange colour changes to green.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
