1
Question
Why the shape of molecule is linear?
Why the shape of molecule is linear?
Open in App
Solution
Step 1: Formula used for calculation of hybridization:
- Where V is the number of valence electrons, M is the monovalent electron, C is the positive charge and A represents the negative charge.
Step 2: Calculation for Hybridisation of :
- The total number of valence electrons on Beryllium in is and there are monovalent atoms.
- The steric number is , thus the hybridization of is .
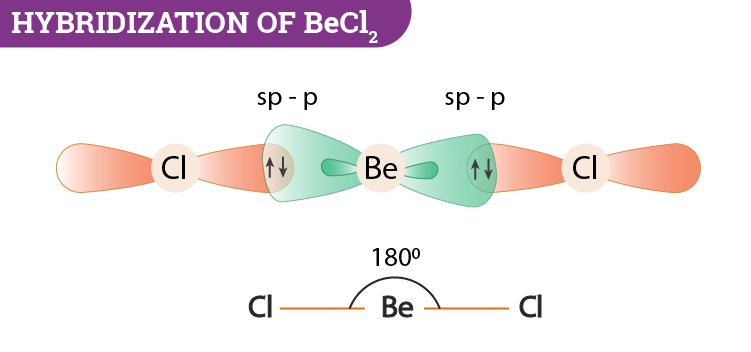
Step 3: Shape of Beryllium chloride:
- Beryllium chloride is a molecule with formula .
- During the formation of , the beryllium atom is covalently bonded with two chlorine atoms.
- In this molecule, there are two bond pairs while no lone pair is found in the molecule.
- The hybridization of is hybridization.
- Thus, as per VSEPR theory, the shape of is linear.
Step 1: Formula used for calculation of hybridization:
- Where V is the number of valence electrons, M is the monovalent electron, C is the positive charge and A represents the negative charge.
Step 2: Calculation for Hybridisation of :
- The total number of valence electrons on Beryllium in is and there are monovalent atoms.
- The steric number is , thus the hybridization of is .
Step 3: Shape of Beryllium chloride:
- Beryllium chloride is a molecule with formula .
- During the formation of , the beryllium atom is covalently bonded with two chlorine atoms.
- In this molecule, there are two bond pairs while no lone pair is found in the molecule.
- The hybridization of is hybridization.
- Thus, as per VSEPR theory, the shape of is linear.

Suggest Corrections
11
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
