1
Question
Three chemical reactions are shown.
1 catalytic addition of steam to ethene
2 combustion of ethanol
3 fermentation of glucose
In which of the reactions does the relative molecular mass of the carbon-containing compound
decrease?
Three chemical reactions are shown.
1 catalytic addition of steam to ethene
2 combustion of ethanol
3 fermentation of glucose
In which of the reactions does the relative molecular mass of the carbon-containing compound
decrease?
Open in App
Solution
The correct option is D 2 and 3
The reaction of ethene with steam to form ethanol can be reversed. This allows ethanol to be converted into ethene. A catalyst of hot aluminium oxide is used to speed up the reaction.
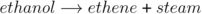
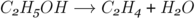
This is called a dehydration reaction.
The reaction of ethene with steam to form ethanol can be reversed. This allows ethanol to be converted into ethene. A catalyst of hot aluminium oxide is used to speed up the reaction.
This is called a dehydration reaction.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program

