1
Question
Three moles of an ideal gas (CP=72R) at pressure, PA and temperature TA is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally gas is compressed at constant volume to its original pressure PA.
The correct P−V and P−T diagrams for the complete process are
Three moles of an ideal gas (CP=72R) at pressure, PA and temperature TA is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally gas is compressed at constant volume to its original pressure PA.
The correct P−V and P−T diagrams for the complete process are
Open in App
Solution
The correct option is C 
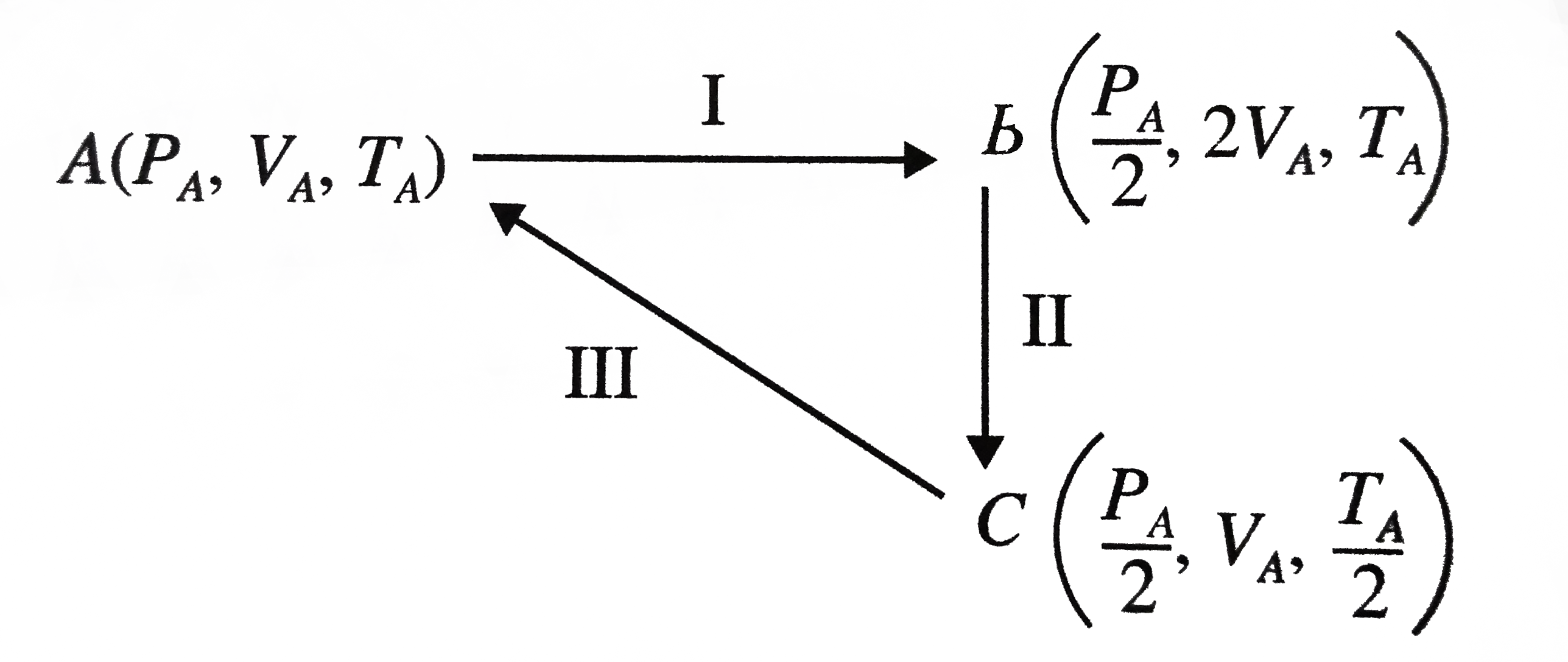
Let the process start from initial pressure PA, volume VA and temperature TA.
I: Isothermal expansion (PV=constant ) at temperature TA to twice the initial volume VA
II: Compression at constant pressure PA/2 to original volume VA (i.e. V ∝ T)
III: Isochoric process (at volume VA) to initial conditions (i.e. P ∝ T )
Why this question?
Importance in JEE: graph based questions involving different processes are frequently asked in JEE.

Let the process start from initial pressure PA, volume VA and temperature TA.
I: Isothermal expansion (PV=constant ) at temperature TA to twice the initial volume VA
II: Compression at constant pressure PA/2 to original volume VA (i.e. V ∝ T)
III: Isochoric process (at volume VA) to initial conditions (i.e. P ∝ T )

| Why this question? Importance in JEE: graph based questions involving different processes are frequently asked in JEE. |
Suggest Corrections
0



