1
Question
Two reactions R1 and R2 have identical pre-exponential factors. Activation energy of R1 exceeds that of R2 by 10Kjmol−1 If K1 and K2 are rate constants for reactions R1 and R2 respectively at 300K, then in(K2/K1) is equal to: (R=8.314 j mol−1K−1)
Two reactions R1 and R2 have identical pre-exponential factors. Activation energy of R1 exceeds that of R2 by 10Kjmol−1 If K1 and K2 are rate constants for reactions R1 and R2 respectively at 300K, then in(K2/K1) is equal to: (R=8.314 j mol−1K−1)
Open in App
Solution
The correct option is C 4
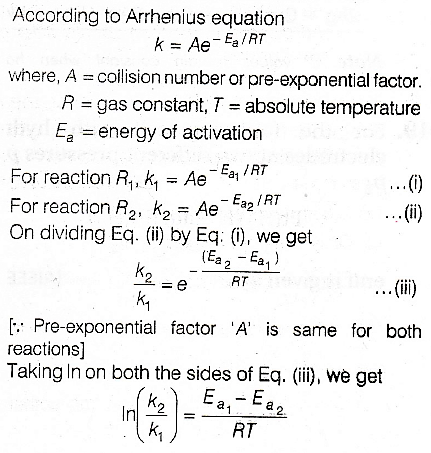

Option C is correct
Option C is correct
Suggest Corrections
1
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
