1
Question
What is the percentage of character in hybridized orbital?
What is the percentage of character in hybridized orbital?
Open in App
Solution
- hybridization consists of one orbital and three orbitals to create a hybrid orbital
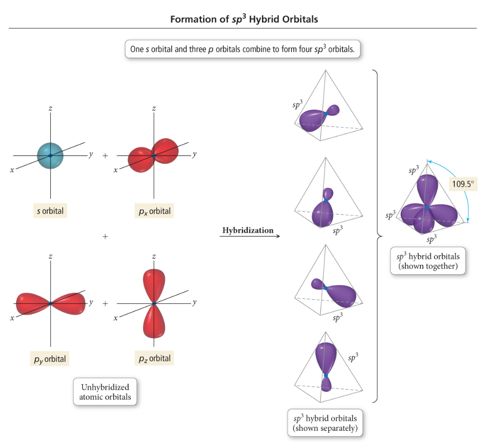
- It has a bond angle and has a geometry of tetrahedral shown in the figure above.
- Since one part is and three parts are orbital, the percentage of is and of is.
- The more the character, the more will be the electronegativity percentage in that hybridization.
Hence, the percentage of .
- hybridization consists of one orbital and three orbitals to create a hybrid orbital

- It has a bond angle and has a geometry of tetrahedral shown in the figure above.
- Since one part is and three parts are orbital, the percentage of is and of is.
- The more the character, the more will be the electronegativity percentage in that hybridization.
Hence, the percentage of .
Suggest Corrections
4
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
