1
Question
What type of intermolecular force exists between polar molecules?
What type of intermolecular force exists between polar molecules?
Open in App
Solution
The correct option is C Dipole-dipole.
Dipole-dipole forces are attractive forces between the positive end
of one polar molecule and the negative end of another
polar molecule. Dipole-dipole forces have strengths that range from
5 kJ to 20 kJ per mole. They are much weaker than ionic or covalent
bonds and have a significant effect only when the molecules involved are
close together (touching or almost touching).
The figures show two arrangements of polar iodine
monochloride (ICl) molecules that give rise to dipole-dipole attractions
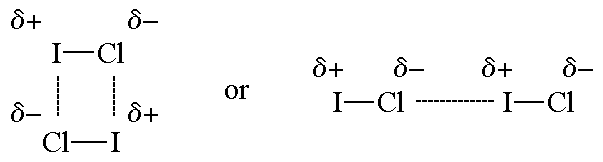
Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. Dipole-dipole forces have strengths that range from 5 kJ to 20 kJ per mole. They are much weaker than ionic or covalent bonds and have a significant effect only when the molecules involved are close together (touching or almost touching).
The figures show two arrangements of polar iodine monochloride (ICl) molecules that give rise to dipole-dipole attractions
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
