1
Question
Which molecule has a trigonal planar geometry?
Which molecule has a trigonal planar geometry?
Open in App
Solution
The correct option is D BF3
IF3 molecule is pyramidal in shape and is sp3 hybridised.

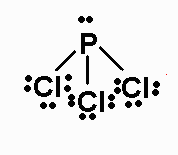
PCl3 molecule is pyramidal in shape and is sp3 hybridised.
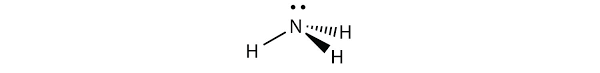
NH3 molecule is pyramidal in shape and is sp3 hybridised.
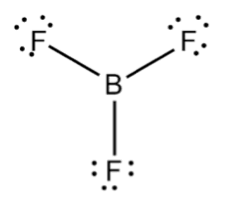
BF3 molecule' is trigonal planar in geometry and is sp2 hybridised.
So, the answer is D.
IF3 molecule is pyramidal in shape and is sp3 hybridised.

PCl3 molecule is pyramidal in shape and is sp3 hybridised.
NH3 molecule is pyramidal in shape and is sp3 hybridised.
BF3 molecule' is trigonal planar in geometry and is sp2 hybridised.
So, the answer is D.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
