1
Question
Which of the following chemical compound is planar?
Which of the following chemical compound is planar?
Open in App
Solution
The correct option is D
Expalantion for correct option:
D.
- is a square planar molecule having two nonbonding electron pairs, one is located above and one is located below the plane of the molecule.
- In xenon tetrafluoride, there are a total of six electrons in the orbital and two electrons in the orbital.
- There are valence electrons in the compound .
- The structure of the compound is square planar.
- The molecule is hybridized.
- The molecule has bond pairs and lone pairs.
- The structure of is shown below:

Explanation for incorrect options:
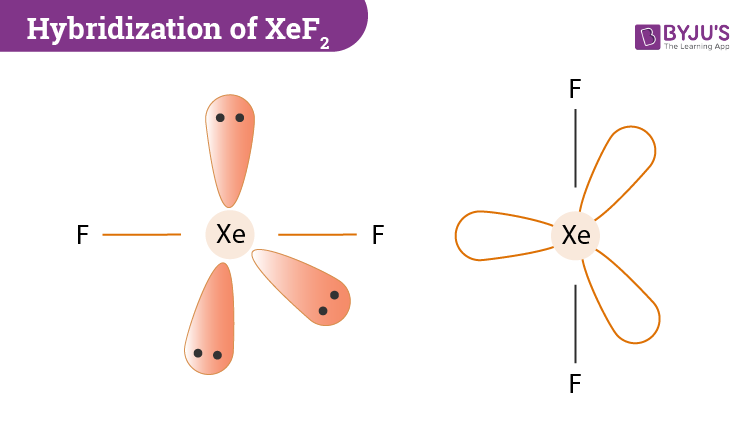
A.
- is a linear molecule having two bonding electron pairs and three non-bonding electron pairs. It has hybridization.
- The two fluorine atoms occupy the axial position of the TBP structure and the three non-bonded pairs occupy the equatorial positions.
- It is a non-planar molecule.
B.
- In , three oxygen atom forms double bonds with xenon while Fluorine forms a single bond.
- A molecule has an unbonded electron and a distorted octahedron structure. It is not planar.

C.
- In , two Oxygen atom forms double bonds with xenon while the two fluorine atom forms a single bond.
- The molecule has an unbonded electron pair and a see-saw structure. It is not planar.

Therefore, the correct option is D. .
Expalantion for correct option:
D.
- is a square planar molecule having two nonbonding electron pairs, one is located above and one is located below the plane of the molecule.
- In xenon tetrafluoride, there are a total of six electrons in the orbital and two electrons in the orbital.
- There are valence electrons in the compound .
- The structure of the compound is square planar.
- The molecule is hybridized.
- The molecule has bond pairs and lone pairs.
- The structure of is shown below:

Explanation for incorrect options:
A.
- is a linear molecule having two bonding electron pairs and three non-bonding electron pairs. It has hybridization.
- The two fluorine atoms occupy the axial position of the TBP structure and the three non-bonded pairs occupy the equatorial positions.
- It is a non-planar molecule.

B.
- In , three oxygen atom forms double bonds with xenon while Fluorine forms a single bond.
- A molecule has an unbonded electron and a distorted octahedron structure. It is not planar.

C.
- In , two Oxygen atom forms double bonds with xenon while the two fluorine atom forms a single bond.
- The molecule has an unbonded electron pair and a see-saw structure. It is not planar.

Therefore, the correct option is D. .
Suggest Corrections
1
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
