1
Question
Which of the following has a bond formed from the transfer of electrons?
Which of the following has a bond formed from the transfer of electrons?
Open in App
Solution
The correct option is B NaCl
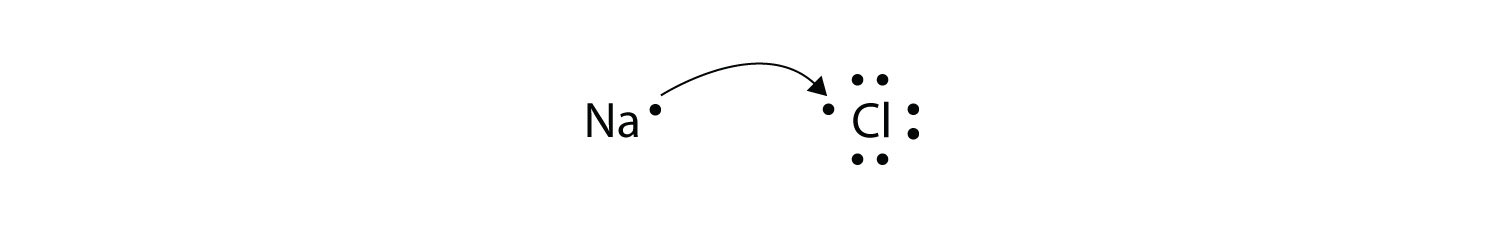
Consider an Na atom in the presence of a Cl atom. The two atoms have
these Lewis electron dot diagrams and electron configurations:

For the Na atom to obtain an octet, it must lose an electron for the Cl
atom to gain an octet, it must gain an electron. An electron transfers
from the Na atom to the Cl atom.
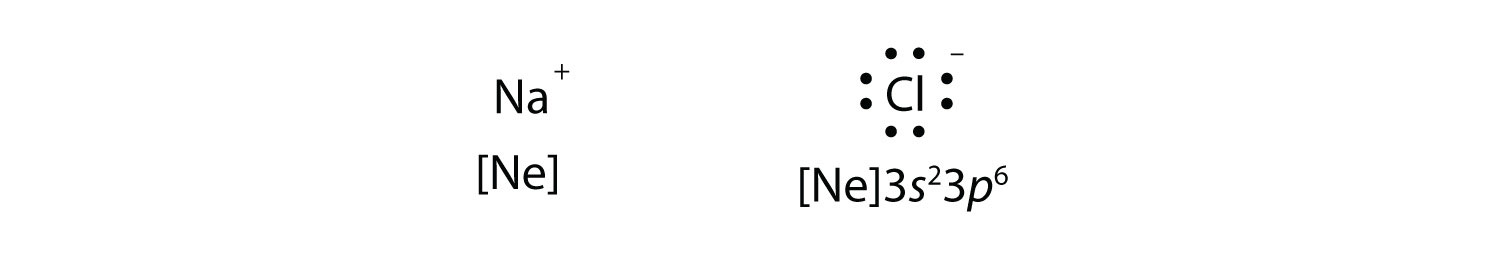
Both species now have complete octets, and the electron shells are
energetically stable. From basic physics, we know that opposite charges
attract. This is what happens to the Na+ and Cl− ions:
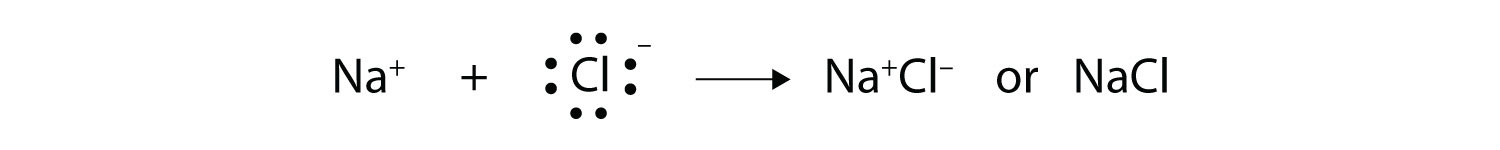
Consider an Na atom in the presence of a Cl atom. The two atoms have these Lewis electron dot diagrams and electron configurations:

For the Na atom to obtain an octet, it must lose an electron for the Cl atom to gain an octet, it must gain an electron. An electron transfers from the Na atom to the Cl atom.

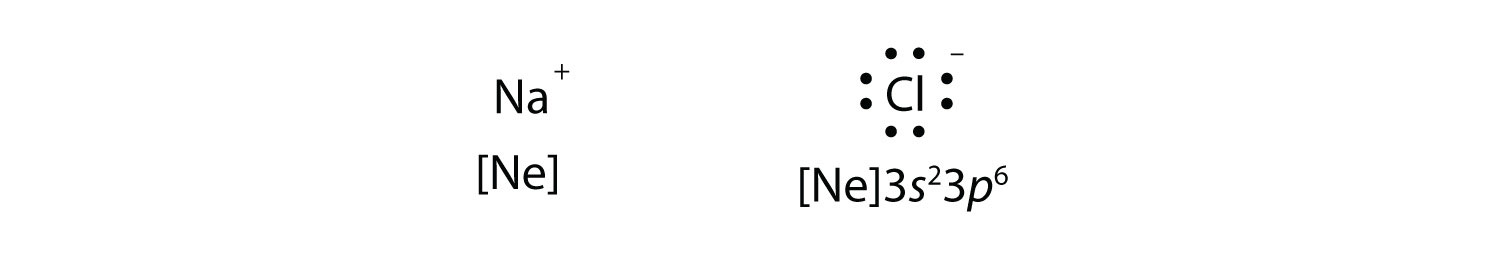
Both species now have complete octets, and the electron shells are energetically stable. From basic physics, we know that opposite charges attract. This is what happens to the Na+ and Cl− ions:

Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
