1
Question
Which of the following is used for dissolving gold?
Which of the following is used for dissolving gold?
Open in App
Solution
The correct option is D Aqua regia
Aqua Regia is a mixture of nitric acid and hydrochloric acid in the ratio 1:3. It is such a powerful solution that, it can dissolve any substance even gold and platinum.This is because the nitric acid part of it is an excellent oxidising agent whereas HCl gives 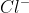 ions which can form a coordination complex with gold or silver ions.
ions which can form a coordination complex with gold or silver ions.
Aqua Regia is a mixture of nitric acid and hydrochloric acid in the ratio 1:3. It is such a powerful solution that, it can dissolve any substance even gold and platinum.
This is because the nitric acid part of it is an excellent oxidising agent whereas HCl gives 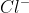 ions which can form a coordination complex with gold or silver ions.
ions which can form a coordination complex with gold or silver ions.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
