1
Question
Which of the following salt will form a basic solution upon hydrolysis?
Which of the following salt will form a basic solution upon hydrolysis?
Open in App
Solution
The correct option is A NaC2H3O2
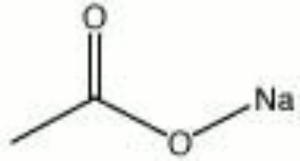
This is CH3COONa salt, which is called a Sodium acetate.
It is made up of acetic acid and NaOH base through neutralisation.
Acetic acid ( CH3COOH ) is a weak acid whereas NaOH is a strong base.
Being weak acid, the acetic acid is formed by the association of acetate ion and H+ thus produced , due to the hydrolysis of CH3COONa.
Hence , there are Na+ and OH− ions in solution making the solution basic (alkaline).
CH3COONa+H2O→CH3COOH+Na++OH−

This is CH3COONa salt, which is called a Sodium acetate.
It is made up of acetic acid and NaOH base through neutralisation.
Acetic acid ( CH3COOH ) is a weak acid whereas NaOH is a strong base.
Being weak acid, the acetic acid is formed by the association of acetate ion and H+ thus produced , due to the hydrolysis of CH3COONa.
Hence , there are Na+ and OH− ions in solution making the solution basic (alkaline).
CH3COONa+H2O→CH3COOH+Na++OH−
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
