1
Question
Which of the following substances yield less than 4 Kcal/mol when its phosphate bond is hydrolysed?
Which of the following substances yield less than 4 Kcal/mol when its phosphate bond is hydrolysed?
Open in App
Solution
The correct option is B Glucose-6-phosphate
The process of glycolysis involves the conversion of glucose to glucose-6-phosphate. The reaction can be written as follows: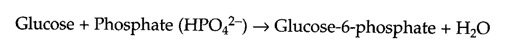
The reaction which the hydrolysis of the phosphate bond in ATP takes place gives rise to free energy range of 7.3 kcal/mol, when ADP is hydrolysed, the reaction also gives free energy in the range of 7.3 kcal/mol. Creatine Phosphate is also a high energy phosphate compound. The reason for high free energy is due to difference of free energy contents in reactants and products. However, the low energy phosphates include glucose-1-P, fructose-6-P, glucose-6-P and AMP. Hydrolysis of Glucose 6-P gives an yield of -3.3 kcal/mol
The process of glycolysis involves the conversion of glucose to glucose-6-phosphate. The reaction can be written as follows:
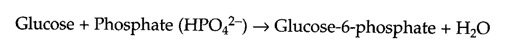
The reaction which the hydrolysis of the phosphate bond in ATP takes place gives rise to free energy range of 7.3 kcal/mol, when ADP is hydrolysed, the reaction also gives free energy in the range of 7.3 kcal/mol. Creatine Phosphate is also a high energy phosphate compound. The reason for high free energy is due to difference of free energy contents in reactants and products. However, the low energy phosphates include glucose-1-P, fructose-6-P, glucose-6-P and AMP. Hydrolysis of Glucose 6-P gives an yield of -3.3 kcal/mol
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
