1
Question
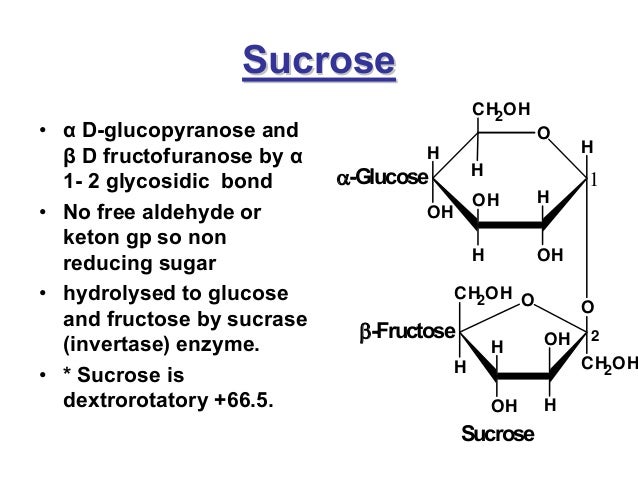
Why sucrose is a non reducing sugar? show it and describe by a diagram?
Open in App
Solution
Non-reducing agents don't have free ketone or aldehyde groups, and therefore contain an acetal instead of a hemiacetal. An acetal has two O-R groups, one –R group and a –H atom attached to the same carbon. (The key difference between an acetal and a hemiactal is that in a hemiacetal, an –OH group replaces one of the –OR acetal groups.) A sugar without a hemiacetal is non-reducing because it does not behave as a reducing agent toward oxidizing metal salts. Sucrose is one example of a non-reducing sugar

Suggest Corrections
2
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
