1
Question
Why the ease of dehydration of alcohols follows a sequence, tertiary secondary primary?
Why the ease of dehydration of alcohols follows a sequence, tertiary secondary primary?
Open in App
Solution
Dehydration of alcohols:
- When alcohol reacts with protic acids, it tends to lose a water molecule, resulting in the formation of alkenes.
- These reactions are known as dehydrogenation or alcohol dehydration.
- It is an illustration of an elimination reaction.
- Example of dehydration of ethanol.
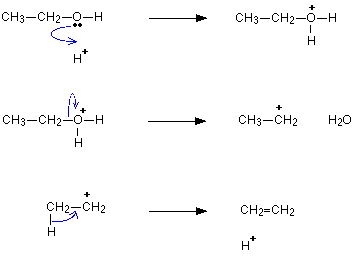
Reason for the ease of dehydration of alcohol following a sequence:
- Alcohols dehydrate easily in the following order: tertiarysecondary primary.
- The stability of the intermediate carbocation can be used to justify the ease with which alcohols can be dehydrated.
- The higher the stability of the produced carbonation, the faster the reaction.

- This is owing to the alkyl group's electron-releasing () action.
Alcohols dehydrate easily in the following order: tertiarysecondary primary, due to the order of stability of carbocations produced.
Dehydration of alcohols:
- When alcohol reacts with protic acids, it tends to lose a water molecule, resulting in the formation of alkenes.
- These reactions are known as dehydrogenation or alcohol dehydration.
- It is an illustration of an elimination reaction.
- Example of dehydration of ethanol.
Reason for the ease of dehydration of alcohol following a sequence:
- Alcohols dehydrate easily in the following order: tertiarysecondary primary.
- The stability of the intermediate carbocation can be used to justify the ease with which alcohols can be dehydrated.
- The higher the stability of the produced carbonation, the faster the reaction.
- This is owing to the alkyl group's electron-releasing () action.
Alcohols dehydrate easily in the following order: tertiarysecondary primary, due to the order of stability of carbocations produced.
Suggest Corrections
18
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program

