1
Question
Write a short note on graphite.
Write a short note on graphite.
Open in App
Solution
Graphite
- Graphite is a carbon allotrope that conducts electricity due to electron delocalization above and below the carbon atom planes.
- The mineral graphite is a crystalline form of carbon and is made up of graphene layers layered on top of each other.
- In Graphite, the atoms of carbon are hybridized and aligned in the same plane, producing hexagonal rings.
- There are numerous layers of particles in the rings. The honeycomb layered structure of graphite is distinctive.
- Each layer is framed up of planar hexagonal rings of carbon atoms with a carbon-carbon bond length of pm (picometers) within the layer.
- Three of the four carbon atoms make sigma bonds, while the fourth one forms a pi-bond. Vander Waal forces grip these layers together.
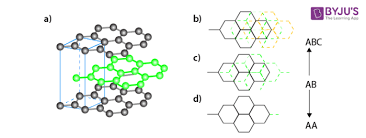
Figure: ABC type of Graphite
Characteristics of Graphite
- It can work as a lubricant as its layers are piled over one other.
- It also has a glossy surface, which contributes to its electrical conductivity
- It is a fantastic heat as well as electricity passager.
- One of the most crucial properties of graphite is that it may be utilized as a parched lubricant in elevated temperature systems where oil cannot be used.
- Graphite is dormant to both acids and alkalis.
Applications of Graphite
- As dispersion material, graphite powder is used as a lubricant.
- In pencils, manufacturing of steel, and mobile phones, graphite can be used.
- As it is an excellent conductor of electricity, it is commonly utilized in the fabrication of carbon electrodes for electrolytic cells.
- Because of its high melting point and inert nature against acids and alkalis, it is used in the manufacturing of crucibles.
- It's found in nuclear reactors and melters.
Graphite
- Graphite is a carbon allotrope that conducts electricity due to electron delocalization above and below the carbon atom planes.
- The mineral graphite is a crystalline form of carbon and is made up of graphene layers layered on top of each other.
- In Graphite, the atoms of carbon are hybridized and aligned in the same plane, producing hexagonal rings.
- There are numerous layers of particles in the rings. The honeycomb layered structure of graphite is distinctive.
- Each layer is framed up of planar hexagonal rings of carbon atoms with a carbon-carbon bond length of pm (picometers) within the layer.
- Three of the four carbon atoms make sigma bonds, while the fourth one forms a pi-bond. Vander Waal forces grip these layers together.
Figure: ABC type of Graphite
Characteristics of Graphite
- It can work as a lubricant as its layers are piled over one other.
- It also has a glossy surface, which contributes to its electrical conductivity
- It is a fantastic heat as well as electricity passager.
- One of the most crucial properties of graphite is that it may be utilized as a parched lubricant in elevated temperature systems where oil cannot be used.
- Graphite is dormant to both acids and alkalis.
Applications of Graphite
- As dispersion material, graphite powder is used as a lubricant.
- In pencils, manufacturing of steel, and mobile phones, graphite can be used.
- As it is an excellent conductor of electricity, it is commonly utilized in the fabrication of carbon electrodes for electrolytic cells.
- Because of its high melting point and inert nature against acids and alkalis, it is used in the manufacturing of crucibles.
- It's found in nuclear reactors and melters.
Suggest Corrections
5
Join BYJU'S Learning Program
Join BYJU'S Learning Program
