1
Question
which type of reaction is this?
which type of reaction is this?
Open in App
Solution
- The given reaction .
- The oxidation state of elements in reaction:
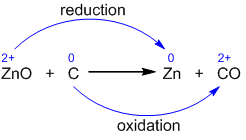
- So, carbon is oxidized because there is an increase in its oxidation state, while zinc is reduced because there is a decrease in its oxidation state.
In the above reaction, carbon is oxidized and zinc is getting reduced simultaneously so it is a type of redox reaction.
- The given reaction .
- The oxidation state of elements in reaction:
- So, carbon is oxidized because there is an increase in its oxidation state, while zinc is reduced because there is a decrease in its oxidation state.
In the above reaction, carbon is oxidized and zinc is getting reduced simultaneously so it is a type of redox reaction.
Suggest Corrections
5
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program

