1
Question
How are the magnitude of repulsion and the distance separating the molecules related?
How are the magnitude of repulsion and the distance separating the molecules related?
Open in App
Solution
The magnitude of repulsion and the distance separating the molecules relation:
- Repulsion is defined as the force which exists between two similar or like entities.
- When two molecules are brought close near to each other, there exists the force of repulsion between the electron clouds and that between the nuclei of two molecules.
- The magnitude of the repulsion is inversely related to the distance separating the molecules as the former increases very rapidly as the distance parting the molecules decreases.
- This is the reason why compression of solid and liquid is a hard process to obtain. As in these states, molecules already exist in close contact with each other.
- Hence, any further compression becomes difficult to proceed, as that would result in the increase of repulsive force of interactions between the molecules.
- The figure showing the relation between the magnitude of repulsion and the distance separating the molecules can be shown as;
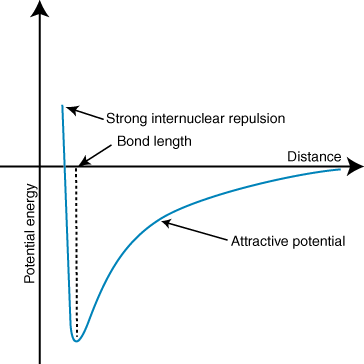
Hence, the magnitude of repulsion and the distance separating the molecules are inversly related to each other as the decrease in distance increases the magnitude of repulsion between the molecules.
The magnitude of repulsion and the distance separating the molecules relation:
- Repulsion is defined as the force which exists between two similar or like entities.
- When two molecules are brought close near to each other, there exists the force of repulsion between the electron clouds and that between the nuclei of two molecules.
- The magnitude of the repulsion is inversely related to the distance separating the molecules as the former increases very rapidly as the distance parting the molecules decreases.
- This is the reason why compression of solid and liquid is a hard process to obtain. As in these states, molecules already exist in close contact with each other.
- Hence, any further compression becomes difficult to proceed, as that would result in the increase of repulsive force of interactions between the molecules.
- The figure showing the relation between the magnitude of repulsion and the distance separating the molecules can be shown as;

Hence, the magnitude of repulsion and the distance separating the molecules are inversly related to each other as the decrease in distance increases the magnitude of repulsion between the molecules.
Suggest Corrections
0
Join BYJU'S Learning Program
Join BYJU'S Learning Program
