1
Question
The bond order of molecule on the basis of molecular orbital theory is ?
The bond order of molecule on the basis of molecular orbital theory is ?
Open in App
Solution
The correct option is C
Explanation for the correct option:
Option (C):
- Bond order is defined as, the number of bonds formed between the atoms in the compound.
- We know that molecular orbital theory (MOT) explains the formation of molecules, which also helps in determining the bond order of the molecule.
- The bond order formula derived from MOT can be given as:
- The electronic configuration of (14 electrons) can be given as:
(Where, and represents the bonding molecular orbitals and , represents the antibonding molecular orbitals ) - The MO diagram of can be given as:
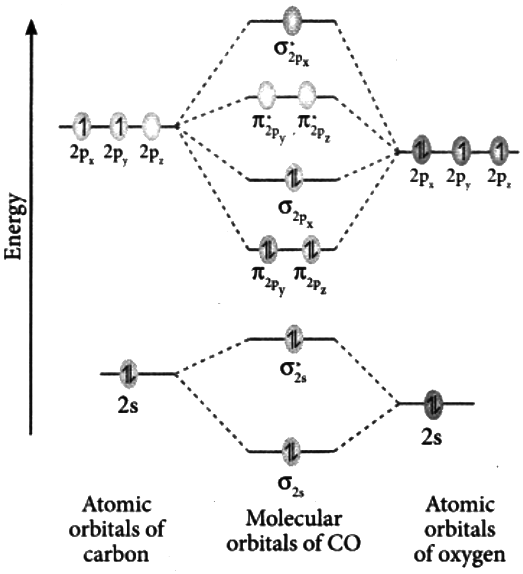
- We can see from the diagram that, and
- Therefore, the Bond order of can be calculated as:
Hence, the bond order of molecule on the basis of the molecular orbital theory is .
Explanation for the correct option:
Option (C):
- Bond order is defined as, the number of bonds formed between the atoms in the compound.
- We know that molecular orbital theory (MOT) explains the formation of molecules, which also helps in determining the bond order of the molecule.
- The bond order formula derived from MOT can be given as:
- The electronic configuration of (14 electrons) can be given as:
(Where, and represents the bonding molecular orbitals and , represents the antibonding molecular orbitals ) - The MO diagram of can be given as:
- We can see from the diagram that, and
- Therefore, the Bond order of can be calculated as:
Hence, the bond order of molecule on the basis of the molecular orbital theory is .
Suggest Corrections
34
Join BYJU'S Learning Program
Join BYJU'S Learning Program
