1
Question
What is a Gold foil experiment?
What is a Gold foil experiment?
Open in App
Solution
Gold-foil experiment:
- The gold foil experiment was designed by Rutherford.
- In his experiment, the particles were made to come down on a thin gold foil.
- Alpha particles are made up of two protons and two neutrons tightly bound together which is identical to Helium-4.
- Many of the particles passed linearly through the gold foil.
- Some of the particles deviated at small angles.
- One out of every particles appeared to bounce.
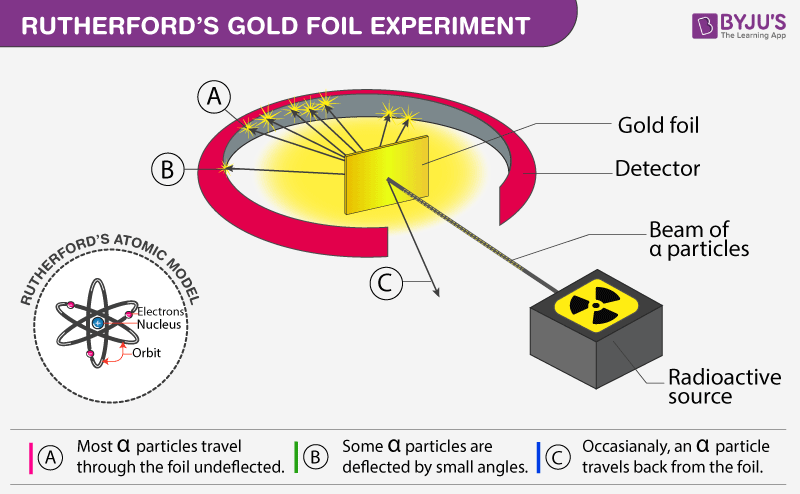
Conclusion of Rutherford's model of an atom:
- The space inside an atom is empty as most of the particles passed without deflection through the gold foil.
- The positive charge occupies a minimum space which indicates that very few particles were diverted from their path.
- A very small proportion of particles were diverted by .

Gold-foil experiment:
- The gold foil experiment was designed by Rutherford.
- In his experiment, the particles were made to come down on a thin gold foil.
- Alpha particles are made up of two protons and two neutrons tightly bound together which is identical to Helium-4.
- Many of the particles passed linearly through the gold foil.
- Some of the particles deviated at small angles.
- One out of every particles appeared to bounce.

Conclusion of Rutherford's model of an atom:
- The space inside an atom is empty as most of the particles passed without deflection through the gold foil.
- The positive charge occupies a minimum space which indicates that very few particles were diverted from their path.
- A very small proportion of particles were diverted by .

Suggest Corrections
40
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
