1
Question
How does the quantum theory explain the photoelectric effect?
How does the quantum theory explain the photoelectric effect?
Open in App
Solution
Explanation:
- According to quantum theory, light is in the form of packets of energy called photons. The energy of the photon is equal to . Here, is the Planck's constant and is the frequency of the photon.
- The emission of electrons when light is shone onto a substance is known as the photoelectric effect. These electrons are referred to as photo-electrons.
- Based on quantum theory, in the photoelectric effect only discrete amounts of energy, known as quanta, can be absorbed or released by electrons (packets).
- Photon's energy is precisely proportional to the frequency of the radiation.
Photon Energy,
Planck's Constant
Radiation
Photoelectric Effect:
- Electrons of a strongly bonded metal are released when incoming light radiation with energy higher than the metal's threshold value strikes the surface.
- The photon is a small piece of light. A photon transfers its total energy upon hitting an electron, causing the electron to drop off the surface.
- A free negative charge known as a photoelectron is created from the remaining energy of the photon.
The Photoelectric Effect Explained by Einstein:
- The intensity of the incident radiation determines the strength of the photoelectric current, which must exceed the threshold frequency.
- This phenomenon is unaffected by the strength of the radiation that occurred.
- The photoelectric effect happens instantly. The metal's electrons leave the surface as soon as light strikes it.
Where '' is Planck's constant, and '' is the frequency of the emitted radiation.
Diagram of photoelectric effect:
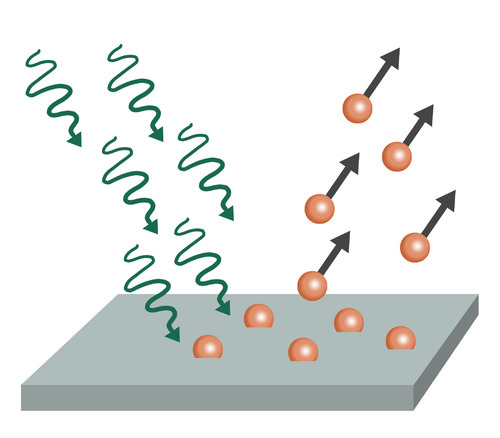
Explanation:
- According to quantum theory, light is in the form of packets of energy called photons. The energy of the photon is equal to . Here, is the Planck's constant and is the frequency of the photon.
- The emission of electrons when light is shone onto a substance is known as the photoelectric effect. These electrons are referred to as photo-electrons.
- Based on quantum theory, in the photoelectric effect only discrete amounts of energy, known as quanta, can be absorbed or released by electrons (packets).
- Photon's energy is precisely proportional to the frequency of the radiation.
Photon Energy,
Planck's Constant
Radiation
Photoelectric Effect:
- Electrons of a strongly bonded metal are released when incoming light radiation with energy higher than the metal's threshold value strikes the surface.
- The photon is a small piece of light. A photon transfers its total energy upon hitting an electron, causing the electron to drop off the surface.
- A free negative charge known as a photoelectron is created from the remaining energy of the photon.
The Photoelectric Effect Explained by Einstein:
- The intensity of the incident radiation determines the strength of the photoelectric current, which must exceed the threshold frequency.
- This phenomenon is unaffected by the strength of the radiation that occurred.
- The photoelectric effect happens instantly. The metal's electrons leave the surface as soon as light strikes it.
Where '' is Planck's constant, and '' is the frequency of the emitted radiation.
Diagram of photoelectric effect:
Suggest Corrections
15
Join BYJU'S Learning Program
Join BYJU'S Learning Program
