Relation between P, V, T, Gamma in Adiabatic Proceses
Trending Questions
Q.
A gas is allowed to expand in a well insulated container against constant external pressure of from an initial volume of to a final volume of . The change in internal energy of the gas in joules will be ?
Q. Two moles of an ideal gas (Cv=52R) was compressed adiabatically against constant pressure of 2 atm. The gas was initially at 350 K and 1 atm pressure. The work involve in the process is equal to:
- 250 R
- 300 R
- 380 R
- 500 R
Q. 10 L of a monoatomic ideal gas at 0oC and 5 atm is suddenly released to 1 atm pressure and the gas is expanded adiabatically against the constant pressure. Find the final volume (in L) of the gas.
- 54 L
- 10 L
- 34 L
- 74 L
Q.
In the mixture 2 mole of and 1 mole of is present. Find at 300 K.
6.32
1.58
3.16
10
Q. When 1 mole of a mono atomic ideal gas a T K undergoes an adiabatic change under a constant external pressure of 1 atm, its volume changes from 1L to 2L. The final temperature in Kelvin is:
- T22/3
- T+23×0.0821
- T
- T−23×0.0821
Q. 2 m3 volume of a gas (γ=1.4) at a pressure of 4×105N/m2 is compressed adiabatically so that its volume becomes 0.5 m3. Calculate the work done in process? (Given 41.4=6.9)
- 1.48×106 J
- 4.5×106 J
- 3.28×108 J
- 4.28×107 J
Q. 20 litres of a monoatomic ideal gas at 0oC and 10 atm pressure is suddenly released to 1 atm pressure and the gas expands adiabatically against this constant pressure. The volume of the gas respectively are
- V=164 L
- V=57 L
- V=123.7 L
- V=68.3 L
Q. Consider a spherical shell of radius R at temperature T. The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume u=UV∝T4 and pressure P=13(UV). If the shell now undergoes an adiabatic expansion the relation between T and R is:
- T∝1R
- T∝1R3
- T∝ e−R
- T∝e−3R
Q. One mol of an ideal gas is allowed to expand reversibly and adiabatically from a temperature of 27oC. If the work done by gas during the process is 2 kJ, then the final temperature of gas is:
Given: Cv for the gas = 20 J/K/mol
Given: Cv for the gas = 20 J/K/mol
- 100 K
- 200 K
- 195 K
- 255 K
Q. Two moles of an ideal gas (Cv=52R) was compressed adiabatically against constant pressure of 2 atm. The gas was initially at 350 K and 1 atm pressure. The work involve in the process is equal to:
- 250 R
- 300 R
- 380 R
- 500 R
Q. P-V plots for two gases during adibatic processes are shown in the following figure. Plots 1 and 2 should correspond respectively to:


- He and O2
- O2 and He
- He and Ar
- O2 and N2
Q. A gas (Cv, m=52R) behaving ideally was allowed to expand reversibly and adiabatically from 1 litre to 32 litre. It's initial temperature was 327oC. The molar enthalpy change (in J mole−1) for the process is
- −1125 R
- −575 R
- −1575 R
- None of these
Q. p-V plots for two gases during adiabatic processes are shown in the following figure. Plots 1 and 2 should correspond respectively to
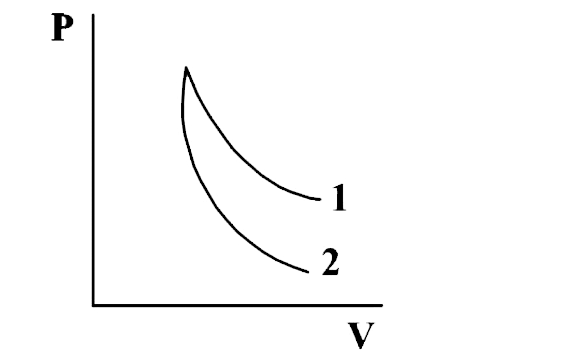

- He and O2
- O2 and He
- He and Ar
- O2 and N2
Q. For a reversible adiabatic ideal gas expansion, the value of dPP is equal to:
- γdVV
- −γdVV
- (γγ−1)dVV
- dVV
Q. 10 L of a monoatomic ideal gas at 0oC and 5 atm is suddenly released to 1 atm pressure and the gas is expanded adiabatically against the constant pressure. Find the final volume (in L) of the gas.
- 54 L
- 10 L
- 34 L
- 74 L
Q. 20 litres of a monoatomic ideal gas at 0oC and 10 atm pressure is suddenly released to 1 atm pressure and the gas expands adiabatically against this constant pressure. The volume of the gas respectively are
- V=164 L
- V=57 L
- V=123.7 L
- V=68.3 L
Q. Consider a spherical shell of radius R at temperature T. The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume u=UV∝T4 and pressure P=13(UV). If the shell now undergoes an adiabatic expansion the relation between T and R is:
- T∝1R
- T∝1R3
- T∝ e−R
- T∝e−3R
Q. The incorrect figure(s) representing isothermal and adiabatic expansion of an ideal gas from a particular initial state is(are):


- A
- B
- C
- D
Q. P-V plots for two gases during adibatic processes are shown in the following figure. Plots 1 and 2 should correspond respectively to:


- He and O2
- O2 and He
- He and Ar
- O2 and N2
Q. 10 L of a monoatomic ideal gas at 0oC and 5 atm is suddenly released to 1 atm pressure and the gas is expanded adiabatically against the constant pressure. Find the final volume (in L) of the gas.
- 54 L
- 10 L
- 34 L
- 74 L
Q. An ideal gas in thermally insulated vessel at internal pressure =P1. volume =V1 and absolute temperature =T1 expands irrversibly against zero external pressure. as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are P2, V2 and T2 respectively. for this expansion.


- q=0
- T2=T1
- P2V2=P1V1
- P2Vγ2=P1Vγ1
Q. If one mole of a monatomic gas (γ=53) is mixed with one mole of a diatomic gas (γ=75) the value of γ for the mixture is:
Q. When 1 mole of a mono atomic ideal gas a T K undergoes an adiabatic change under a constant external pressure of 1 atm, its volume changes from 1L to 2L. The final temperature in Kelvin is:
- T22/3
- T+23×0.0821
- T
- T−23×0.0821
