1
Question
Define monoatomic ion.
Define monoatomic ion.
Open in App
Solution
- Sometimes atoms become charged by losing or gaining electrons.
- By losing electrons, metals form a positively charged ion known as a cation.
- By gaining electrons, nonmetals form a negatively charged ion known as an anion.
- A monoatomic ion also called a simple ion, is an ion consisting of only one atom.
- They are formed by losing or gaining electrons in the outermost shell of an atom.
- There is no chemical bond between the atoms in the ion.
- If an ion contains more than one atom, even though these atoms are from the same element, it is called a polyatomic ion.
Example: In compound consists of monoatomic ion and polyatomic ion .
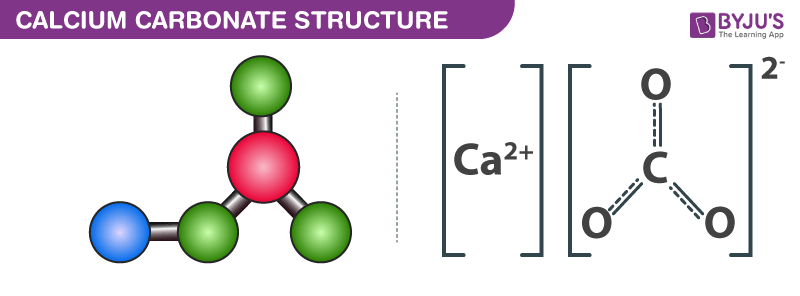
- Sometimes atoms become charged by losing or gaining electrons.
- By losing electrons, metals form a positively charged ion known as a cation.
- By gaining electrons, nonmetals form a negatively charged ion known as an anion.
- A monoatomic ion also called a simple ion, is an ion consisting of only one atom.
- They are formed by losing or gaining electrons in the outermost shell of an atom.
- There is no chemical bond between the atoms in the ion.
- If an ion contains more than one atom, even though these atoms are from the same element, it is called a polyatomic ion.
Example: In compound consists of monoatomic ion and polyatomic ion .

Suggest Corrections
0
