1
Question
Ethylene reacts with bromine to form:
Ethylene reacts with bromine to form:
Open in App
Solution
The correct option is B ethylene dibromide
Mechanism:
The top bromine atom becomes attached to both carbon atoms, with the positive charge being found on the bromine rather than on one of the carbons. A bromonium ion is formed.
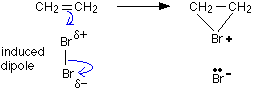
The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction. It can't be attacked by its original bromide ion because the bromonium ion is completely cluttered up with a positive bromine on that side.
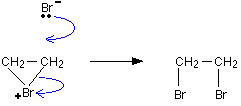
Hence Final product is ethylene Bromide
Mechanism:
The top bromine atom becomes attached to both carbon atoms, with the positive charge being found on the bromine rather than on one of the carbons. A bromonium ion is formed.
The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction. It can't be attacked by its original bromide ion because the bromonium ion is completely cluttered up with a positive bromine on that side.
Hence Final product is ethylene Bromide
Suggest Corrections
1
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
