1
Question
In OF2, number of bond pairs and lone pairs of electrons are respectively:
In OF2, number of bond pairs and lone pairs of electrons are respectively:
Open in App
Solution
The correct option is A 2,8
Explanation:For number of bond pair and lone pair identification we need to draw the structure of OF2, which is as follows: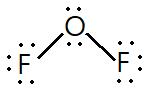 It is clear from the structure that :There are two bond pairs of electrons which forms two O−F bond pairs. O atom has two lone pairs of electron. Each F atom has 3 lone pairs of electrons. Thus in total there are 8 lone pairs of electrons.Hence option is correct
It is clear from the structure that :There are two bond pairs of electrons which forms two O−F bond pairs. O atom has two lone pairs of electron. Each F atom has 3 lone pairs of electrons. Thus in total there are 8 lone pairs of electrons.Hence option is correct
Explanation:
For number of bond pair and lone pair identification we need to draw the structure of OF2, which is as follows:

It is clear from the structure that :There are two bond pairs of electrons which forms two O−F bond pairs. O atom has two lone pairs of electron. Each F atom has 3 lone pairs of electrons. Thus in total there are 8 lone pairs of electrons.
Hence option is correct
Suggest Corrections
2
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
