& are
& are
The correct option is D functional group isomer
Explanation for correct option
(D)functional group isomer
Definition of functional group:
Functional groups are groups of one or more atoms with unique chemical properties.
Definition of functional group isomer:
Functional isomers have the same molecular formula ( the same number of atoms of the same elements) but the atoms are connected to a different functional group, hence showing characteristic properties of distinct groups such as alcohols, carboxylic acids, etc.
is ethyl alcohol.
- Alcohols have the formula
is dimethyl ether
- The general formula of ether is given as
Therefore, they are functional isomers as they have the same molecular formula but different functional groups present in each of them.
Explanation for incorrect option
(A)Position isomer
They have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups inside the molecule

(B)Chain isomer
In chain isomerism, the structure differs in the arrangement of the carbon chains, which may be branched or straight
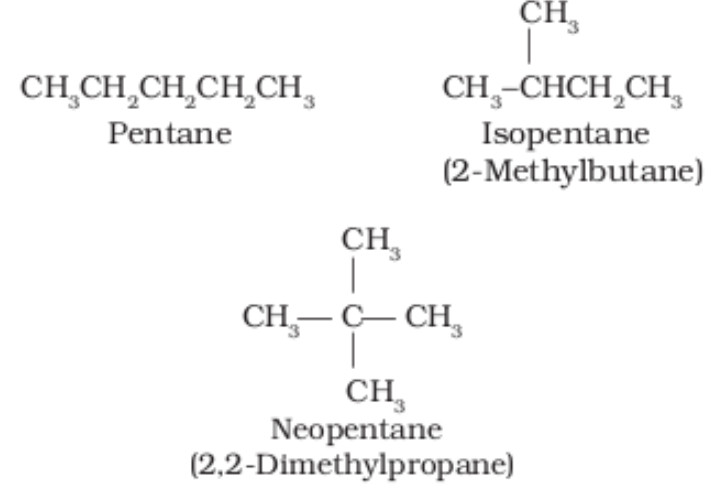
(C)Homologs
A compound belonging to the series of compounds differing by one repeating unit such as .

functional group isomer
Explanation for correct option
(D)functional group isomer
Definition of functional group:
Functional groups are groups of one or more atoms with unique chemical properties.
Definition of functional group isomer:
Functional isomers have the same molecular formula ( the same number of atoms of the same elements) but the atoms are connected to a different functional group, hence showing characteristic properties of distinct groups such as alcohols, carboxylic acids, etc.
is ethyl alcohol.
- Alcohols have the formula
is dimethyl ether
- The general formula of ether is given as
Therefore, they are functional isomers as they have the same molecular formula but different functional groups present in each of them.
Explanation for incorrect option
(A)Position isomer
They have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups inside the molecule

(B)Chain isomer
In chain isomerism, the structure differs in the arrangement of the carbon chains, which may be branched or straight

(C)Homologs
A compound belonging to the series of compounds differing by one repeating unit such as .


.png_img_upload_solution_2022-05-29 04:00:13.276665.png)