1
Question
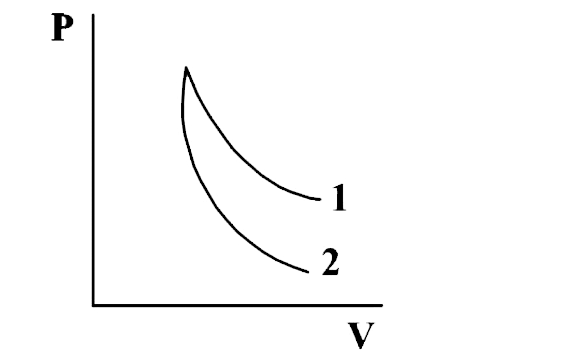
p-V plots for two gases during adibatic processes are shown in the following figure. Plots 1 and 2 should correspond respectively to:

p-V plots for two gases during adibatic processes are shown in the following figure. Plots 1 and 2 should correspond respectively to:

Open in App
Solution
The correct option is B O2 and He
We know, for an adiabatic process, PVγ = constant
where γ = poisson's ratio = CpCv
γ for a monoatomic gas = 53
γ for a diatomic gas = 75
So, γ for a diatomic gas is less than that of monoatomic gas
Now, slope of adiabatic curve is −γP/V
Higher γ implies greater slope.
Hence, curve 1 is for diatomic gas (O2) and curve 2 is for monoatomic gas (He).
We know, for an adiabatic process, PVγ = constant
where γ = poisson's ratio = CpCv
γ for a monoatomic gas = 53
γ for a diatomic gas = 75
So, γ for a diatomic gas is less than that of monoatomic gas
Now, slope of adiabatic curve is −γP/V
Higher γ implies greater slope.
Hence, curve 1 is for diatomic gas (O2) and curve 2 is for monoatomic gas (He).
Suggest Corrections
0
Join BYJU'S Learning Program
Join BYJU'S Learning Program