1
Question
The monobasic acid among the following is:
The monobasic acid among the following is:
Open in App
Solution
The correct option is C H3PO2
ACIDS STRUCTURE H3PO3 (it is dibasic acid since it can release 2 hydrogen ions) 
H2S2O7 (it is a dibasic acid since it can release 2 hydrogen ions ) 
H3PO2 (it is a monobasic since it can release one hydrogen ion) 
H2P2O7 (it is a dibasic since it can release two hydrogen ions) 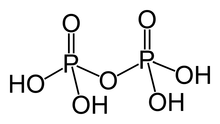
Hence, from the above structures it is clear that H3PO2 is a monobasic acid since it can release only one hydrogen ion.Thus, the correct answer is option (C).
| ACIDS | STRUCTURE |
| H3PO3 (it is dibasic acid since it can release 2 hydrogen ions) |  |
| H2S2O7 (it is a dibasic acid since it can release 2 hydrogen ions ) |  |
| H3PO2 (it is a monobasic since it can release one hydrogen ion) |  |
| H2P2O7 (it is a dibasic since it can release two hydrogen ions) |  |
Thus, the correct answer is option (C).
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
