1
Question
What are the most probable velocity and the average velocity for a system that follows the Maxwell-Boltzmann distribution?
What are the most probable velocity and the average velocity for a system that follows the Maxwell-Boltzmann distribution?
Open in App
Solution
Maxwell-Boltzmann distribution:
- The Maxwell-Boltzmann distribution is frequently depicted in the graph below.
- The y-axis of the Maxwell-Boltzmann graph represents the number of molecules per unit speed.
- So, if the graph in a specific region is higher, it suggests there are more gas molecules traveling at those rates.
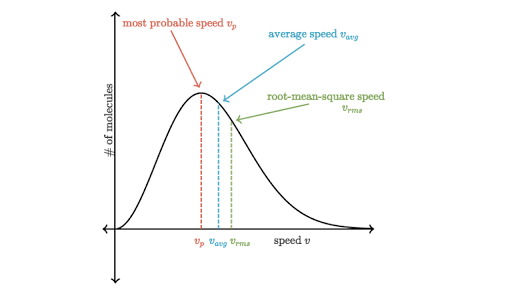
Most probable velocity :
- The most likely velocity is the velocity possessed by the greatest fraction of molecules at the same temperature is most probable velocity.
- Here, R is the gas constant, T is the temperature, and, M is the molar mass of the gas.
Average velocity :
- The average velocity is the displacement divided by the total time. We compute the expected value of v.
Maxwell-Boltzmann distribution:
- The Maxwell-Boltzmann distribution is frequently depicted in the graph below.
- The y-axis of the Maxwell-Boltzmann graph represents the number of molecules per unit speed.
- So, if the graph in a specific region is higher, it suggests there are more gas molecules traveling at those rates.

Most probable velocity :
- The most likely velocity is the velocity possessed by the greatest fraction of molecules at the same temperature is most probable velocity.
- Here, R is the gas constant, T is the temperature, and, M is the molar mass of the gas.
Average velocity :
- The average velocity is the displacement divided by the total time. We compute the expected value of v.
Suggest Corrections
6
Join BYJU'S Learning Program
Join BYJU'S Learning Program

